Abstract
Introduction
Minimal residual disease (MRD) measured by multi-color flow cytometry has been associated with an increased risk of relapse in patients with acute myeloid leukemia (AML). There is also a strong interest in using somatic mutation as an MRD marker and persistence of NPM1 mutation after 2 cycles of induction therapy has been shown to increase the risk of relapse in AML (Ivey et al, NEJM 2016). However, only one third of AML patients carry NPM1 mutation and prognostic impact of persistent mutations in the context of other co-occurring mutations and association with flow cytometry measured MRD is not well understood.
Methods
We studied 95 patients with previously untreated AML who received frontline induction chemotherapy and subsequently achieved complete remission (CR). Paired bone marrow samples obtained at pre-treatment and CR (median day 31) were analyzed by targeted capture exome sequencing of 295 genes (median 309x coverage). Mutect and Pindel was used to call single nucleotide variants (SNVs) and small indels, respectively. We defined two levels of mutation clearance (MC) based on the variant allele frequency (VAF) of the detected mutations at CR: 1) MC2.5, if persistent mutation with VAF <2.5%, and 2) MC1.0 if persistent mutation with VAF <1%. Overall survival (OS), relapse free survival (RFS), event free survival (EFS), and cumulative incidence of relapse (CIR) considering non-relapse death as a competing event were calculated from the date of CR.
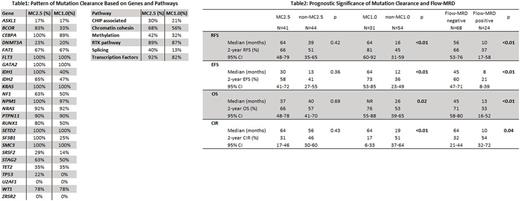
Results
Of 95 patients, 49 (52%) were male and 46 (48%) were female. The median age was 53 (range: 17-76). Conventional karyotyping showed one (1%) patient with favorable cytogenetics (CG), 70 (74%) with intermediate CG, and 21 (22%) with adverse CG (all defined by ELN criteria). Eighty nine (94%) patients had de novo AML and 6 (6%) had secondary AML. Thirty-nine (41%) patients underwent allogeneic stem cell transplant in first CR. In the pre-treatment samples of 95 AML patients, we detected 299 high-confidence somatic mutations (192 SNVs and 107 indels) in 60 genes in 85 (89%) patients. The most frequently mutated genes were NPM1 (31%) followed by DNMT3A (29%), FLT3 (18%), and IDH2 (18%). In the matching CR samples, 84 (28%) and 108 (36%) mutations persisted at the level of VAF ≥2.5% and ≥1%, respectively, which corresponded to 41 (48%) and 31 (36%) patients achieving MC2.5 and MC1.0, respectively. Pattern of MC was distinct between mutated genes and affected molecular pathways (Table1). Mutations associated with clonal hematopoiesis of indeterminate potential (CHIP), DNA methylation, and splicing had low rate of MC, whereas mutations in transcription factor genes or receptor tyrosine kinase (RTK) pathway had high rate of MC. With the median follow-up of 41 months (95% CI: 39-47), patients who achieved MC1.0 had significantly better RFS, EFS, OS and lower CIR than those without MC1.0, while there was no significant difference in any of the above outcomes by MC2.5 (Table 2). Flow cytometry-based MRD (flow-MRD) data were available in 92 (97%) patients at CR. Positive flow-MRD was associated with significantly worse RFS, EFS, OS and CIR (Table2). Among 61 patients with negative flow-MRD, 25 (41%) and 32 (52%) had persistent mutations with VAF ≥2.5% and ≥1%, respectively. Multivariable analysis considering mutations that were prognostic in univariable analysis (NPM1, IDH1), age, ELN-defined adverse cytogenetics, flow-MRD and MC revealed that MC1.0 was a significant prognostic factor for RFS (p = 0.03, HR 0.36, 95%CI: 0.14-0.91) and CIR (p = 0.03, HR 0.42, 95%CI: 0.19-0.92). MC1.0 in DNA methylation (2-year EFS: 85% (95% CI: 51-96) in MC1.0 vs. 40% (95% CI: 23-56) in non-MC1.0, p = 0.01; 2-year OS: 85% (95% CI: 51-96) in MC1.0 vs. 58% (95% CI: 39-72) in non-MC1.0, p = 0.03) or in chromatin-cohesin pathways (2-year EFS: 73% (95% CI: 37-90) in MC1.0 vs. 15% (95% CI: 3-39) in non-MC1.0, p < 0.01; 2-year OS: 82% (95% CI: 45-95) in MC1.0 vs. 39% (95% CI: 14-63) in non-MC1.0, p < 0.01) was associated with significantly better EFS and OS.
Conclusion
In patients with AML treated with induction chemotherapy, persistent somatic mutation at CR with VAF >1% was associated with increased risk of death and relapse. Assessment of both mutation clearance and flow-based MRD at CR may help further risk stratification of AML.
Daver: Immunogen: Research Funding; Kiromic: Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Incyte Corporation: Honoraria, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Otsuka America Pharmaceutical, Inc.: Consultancy; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Jazz: Consultancy; Pfizer Inc.: Consultancy, Research Funding. Cortes: ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Sun Pharma: Research Funding; BMS: Consultancy, Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Kantarjian: Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; Amgen: Research Funding; Novartis: Research Funding. Takahashi: Symbio Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.